Amlodipine (brand name Norvasc) a calcium channel blocker that dilates (widens) blood vessels and improves blood flow. Amlodipine is used to treat chest pain (angina) and other conditions caused by coronary artery disease. Amlodipine is also used to treat high blood pressure (hypertension). Lowering blood pressure may lower your risk of a stroke or heart attack. Amlodipine is for use in adults and children who are at least 6 years old.
It is available under a number of trade names Katerzia and Norvasc.
Drug Fact
Class : Calcium channel blocker
Category : Prescription Only
Uses : treat chest pain (angina) and other conditions caused by coronary artery disease.
Consumed by : adults and children who are at least 6 years old.
Pregnancy category : C
Category C: Either studies in animals have revealed adverse effects on the foetus (teratogenic or embryocidal or other) and there are no controlled studies in women or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the foetus.
Dosage form : tablet
Administration
May be taken with or without food.
Contraindications
Severe hypotension, cardiogenic shock, left ventricular outflow tract obstruction (e.g. high-grade aortic stenosis), heart failure after acute MI.
Special Precautions
Patients with aortic stenosis, congestive heart failure, hypertrophic cardiomyopathy, outflow tract obstruction, severe obstructive coronary disease. Hepatic impairment. Elderly and children. Pregnancy and lactation.
Adverse Reactions
Significant: Peripheral oedema, hypotension, angina/MI.
Blood and lymphatic system disorders: Leucopenia, thrombocytopenia.
Cardiac disorders: Bradycardia, ventricular tachycardia, chest pain, palpitation.
Ear and labyrinth disorders: Tinnitus.
Eye disorders: Visual disturbance, diplopia, conjunctivitis.
Gastrointestinal disorders: Nausea, dyspepsia, diarrhoea, constipation, abdominal pain.
General disorders and admin site conditions: Fatigue, asthenia.
Investigations: Weight gain, elevated hepatic enzymes.
Musculoskeletal and connective tissue disorders: Muscle cramps, arthralgia, back pain.
Nervous system disorders: Headache, somnolence, dizziness, extrapyramidal symptoms.
Psychiatric disorders: Insomnia, anxiety, depression.
Renal and urinary disorders: Micturition disorder, nocturia.
Reproductive system and breast disorders: Sexual dysfunction, gynaecomastia.
Respiratory, thoracic and mediastinal disorders: Pulmonary oedema, dyspnoea, cough, rhinitis.
Skin and subcutaneous tissue disorders: Rash, skin discolouration, pruritus.
Vascular disorders: Flushing.
Pregnancy Category (US FDA)
PO: C
Patient Counseling Information
This drug may cause dizziness, headache, fatigue or nausea, if affected, do not drive or operate machinery.
Monitoring Parameters
Monitor BP, heart rate, pulse, frequency and intensity of angina, weight, and peripheral oedema.
Overdosage
Symptoms: Bradycardia, dysrhythmia, marked hypotension, excessive peripheral vasodilation, reflex tachycardia, shock. Management: Perform gastric lavage and administration of charcoal up to 2 hours after ingestion. Initiate active cardiovascular support, monitor cardiac and respiratory function, elevation of extremities and attention of circulation fluid volume and urine output. For restoring vascular tone and blood pressure, may give a vasoconstrictor and IV Ca gluconate to reverse the effects of Ca channel blocker.
Drug Interactions
Increased systemic plasma concentration with immunosuppressants (e.g. ciclosporin, tacrolimus). Increased serum concentration of simvastatin. Increased exposure with CYP3A4 enzyme inhibitors (e.g. protease inhibitors, azole antifungals, erythromycin, diltiazem). Decreased plasma concentration with CYP3A4 inducers (e.g. rifampicin).
Food Interaction
Increased plasma concentration with grapefruit or grapefruit juice. Decreased plasma concentrations with St. John’s wort.
Lab Interference
May result to false-positive aldosterone/renin ratio (ARR).
Action
Description: Amlodipine, a dihydropyridine Ca-channel blocker, reduces peripheral vascular resistance and BP by relaxing coronary vascular smooth muscle and coronary vasodilation through inhibition of Ca ion transmembrane influx into cardiac and vascular smooth muscles.
Onset: 24-48 hours.
Duration: 24 hours.
Pharmacokinetics:
Absorption: Well absorbed from the gastrointestinal tract. Bioavailability: Approx 60-65%. Time to peak plasma concentration: 6-12 hours.
Distribution: Crosses placenta and enters breast milk. Volume of distribution: 21 L/kg. Plasma protein binding: Approx 98%.
Metabolism: Extensively metabolised in the liver to inactive metabolites.
Excretion: Via urine (60% as metabolites, 10% as unchanged drug). Terminal elimination half-life: 35-50 hours.
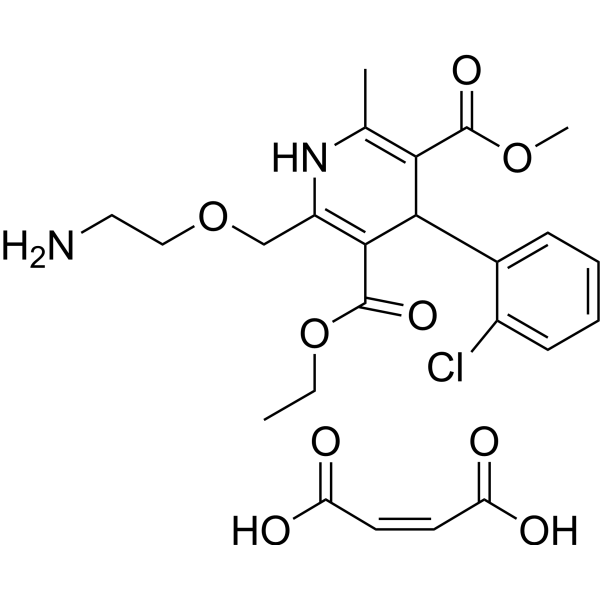
Chemical Structure
Storage
Store between 15-30°C. Protect from light.
ATC Classification
C08CA01 - amlodipine ; Belongs to the class of dihydropyridine derivative selective calcium-channel blockers with mainly vascular effects. Used in the treatment of cardiovascular diseases.



0 Comments